Forensic investigators occasionally need to turn to analytical chemistry to solve a case and effect a recovery or repudiate a claim. An ex-colleague of mine used to jokingly coin the term ‘organoleptic testing,’ or using one’s senses to identify a chemical – by looking at it and sniffing it! It’s a bit old-school and not a health and safety-approved technique. Thankfully, scientific techniques provide much more reliable answers, and I present here a few such techniques and their applications, my webinar on this topic is also available to view.
Chromatography
Many of us may remember chromatography experiments at school. Putting a spot of ink on a strip of blotting paper and dangling it in water, then waiting while the water soaked up the paper, separating the ink into a multitude of colours.
Advanced chromatography techniques are powerful tools for separating mixtures of chemicals for further analysis, and the fundamentals remain the same as in your school experiments. ‘Chromatography’ is somewhat of a misnomer as it doesn’t rely on separating chemicals according to their colour. It relies on the chemical interaction between the sample of interest which is a mixture of chemicals (such as ink), and the two components of the technique which are the ‘stationary phase’ (e.g. the blotting paper), and the ‘mobile phase’ (e.g. the water). The different chemical components of the ink interact preferentially with either the water or the blotting paper. More soluble components will interact preferentially with the water, and less with the blotting paper, moving further along the paper, and vice versa.
High-performance liquid chromatography (HPLC) pushes the basic principles of chromatography to the next level. At the centre of the HPLC is a long, narrow tube, or column. The column is filled with a microporous matrix, which has an enormous surface area. This inert matrix is coated with the stationary phase, generally a chemical selected for its interaction with the sample to be analysed. The sample, dissolved in the mobile phase, is forced into the column at high pressure. The elevated pressure in combination with the high surface area of the stationary phase allows faster processing and leads to better separation of the different chemical components. This would be analogous to a school experiment involving separating an ink-spot on a piece of blotting paper hundreds of metres long, in a matter of minutes. HPLC is used for separating a very wide array of chemicals, such as pharmaceutical products, biological samples, foodstuffs, explosives, narcotics and environmental samples.
Gas Chromatography (GC) uses a very thin glass tube (also called a column) that contains a viscous liquid stationary phase. However, unlike HPLC, the mobile phase is an inert gas, such as helium, nitrogen or argon. The obvious catch to GC is that liquids or solids must be converted to a gaseous state, meaning the sample must be heated up and vapourised. Consequently, GC analysis is generally performed at high temperatures, usually between 50 and 350 °C. GC is particularly useful for detecting volatile contaminants and separating smaller organic molecules, including air monitoring samples, foods, fuels and other accelerants.
Mass Spectrometry
So, your ink-spot on blotting paper has separated nicely, you dry it out and look at the different colours. But how do you analyse the products of GC or HPLC?
As the purified chemicals reach the end of the column, with the least retained chemicals leaving the column (i.e. they are ‘eluted’) first, they can be analysed directly by a variety of techniques.
One of the more commonly used analysis techniques is mass spectrometry (MS) which, as the name suggests, analyses chemicals according to their mass. In most mass spectrometers, the chemical is ionised (or charged), and the ions can then be manipulated by electric and magnetic fields before they arrive at a sensor. Chemicals can then be identified by comparing their mass-to-charge ratio with those of known standards, along with any fragments they may produce during ionisation. The data from MS can be combined with the rate at which the chemicals were eluted through at the chromatography stage, and this can give an accurate picture of the chemical composition of the original sample. The types of chemicals readily separated using HPLC and GC are particularly suited to analysis by MS (commonly referred to as LC-MS or GC-MS), and they are often combined into a single scientific instrument.
Spectroscopy
Spectroscopy is an analytical tool relying on the interaction between chemicals and electromagnetic radiation (e.g. light). All chemicals absorb, to varied extents, light at different wavelengths, even liquids and solutions that appear colourless to the human eye. We cannot see the light at the wavelengths they absorb as these are invisible to the human eye, examples being radio waves, infrared or ultraviolet light, or x-rays.
The interaction of molecules with radio waves is measured using large magnetic fields, such as those in magnetic resonance imaging machines used for medical purposes. Many medical scanners are tuned to detect water (e.g. in tissues), but other chemicals can be detected and measured in specifically designed nuclear magnetic resonance spectrometers.
Spectroscopic methods that measure either infrared (IR) or ultraviolet (UV) light are used to identify molecular or atomic properties of chemicals. The analysis involves passing light through a sample and measuring the frequencies that the sample absorbs. Measurement of the radio properties of a sample can be used for quantification, albeit the technique is not particularly sensitive.
In IR spectroscopy, the properties of the bonds between atoms determines the IR spectrum of a sample. For example, a carbon-oxygen bond will show a different absorption to a carbon-sulphur or carbon-nitrogen bond. A particularly accurate technique used in modern IR spectrometers is Fourier Transform IR spectroscopy which involves bombarding samples with multiple wavelengths of IR light. This improves the sensitivity and speed of analysis. FTIR spectroscopes have been used to distinguish between explosives, solvents, plastics and gas mixtures, amongst other applications. One downside is that IR spectroscopy tends to be difficult to use for quantifying the amount of a specific chemical and is generally used for identification, or semi-quantitative analysis.
UV spectroscopy is often coupled with analysis of the visible spectrum of light. In general, UV analysis tends to be less specific for organic chemicals, but can be utilised effectively for inorganic chemicals, particularly those containing metals. UV light is absorbed by the intra-molecular bonds – those bonds which hold the atoms of a molecule together. Due to the size of the bonds, their interaction with ultraviolet light is particularly distinctive, and this can be measured. The wavelength of UV light absorbed by different types of bonds within a molecule is specific to each bond; for example, there are four chemical bonds in ethanol:
1- Between two Carbon (C) atoms
2- Between Carbon (C) and Hydrogen (H)
3- Between Carbon (C) and Oxygen (O)
4- Between Oxygen (O) and Hydrogen (H)
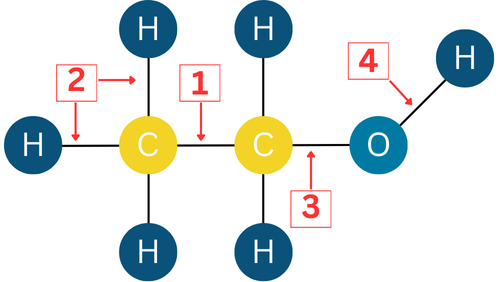
Each of these chemical bonds absorbs a different wavelength of UV light. To add to the complexity, all bonds within a molecule influence each other, and this slightly alters the exact wavelengths absorbed by any of the individual bond. The outcome is that what we might see with the naked eye as a red, green or even a colourless chemical, will have a unique UV absorption ‘fingerprint.’ UV spectroscopy is particularly suited to analysing samples separated by HPLC.
X-radiation (X-ray) is the most intense form of electromagnetic radiation. When chemicals are exposed to X-rays, this ‘excites’ the atoms which make up the chemicals, and they emit further X-rays, at a different wavelength, in response. The ‘fingerprint’ of emitted X-rays is unique to different atoms. X-ray fluorescence spectroscopy (‘XRF’) utilises this effect by measuring the X-rays emitted to provide valuable information on the atomic composition of a sample, but the information must be interpreted carefully because it does not provide much information on the molecular structure of a chemical. XRF is a particularly powerful technique for determining the metal content of a sample, but smaller elements such as carbon, sulphur or phosphorous might not be detected as readily as larger, and therefore heavier, elements such as iron, gold or platinum. XRF is a very easy technique to apply, and Hawkins has recently purchased an XRF analyser which can be used to analyse, for example, the metallurgical composition of structural components and the chemical makeup of contaminants. However, by its nature, XRF can only analyse the surface of a sample.
As is clear from the various advantages and limitations outlined above, each technique has its place, but before undertaking analysis we must be clear of the likely outcomes and how these would impact an opinion formed on the back of these. In many cases, the use of multiple techniques on a sample can provide sufficient data for a useful opinion to be provided, once the analysis is complete. It is also equally important to consider the limitations and what the laboratory tests and data cannot provide.
Examples of Analysis
Accelerants
The analysis techniques described above, offer themselves as valuable tools to forensic investigators. Perhaps the most common requirement by Hawkins Investigators is to establish whether a fire was set intentionally using accelerants. Occasionally at a fire scene, accelerant residues (e.g. petrol) can be smelled if they haven’t been entirely consumed in the fire, for example where they have soaked into a doormat – a good application of organoleptic testing!
I recently investigated a case where the accelerant, easily identifiable as petrol, had soaked under non-combustible floor tiles. However, It is often very difficult to detect anything owing to the overpowering smell of charred debris, so if there is a suspicion that accelerants have been used, a swab can be taken from the area and sent for analysis. FTIR and GC-MS can both detect organic molecules, and GC-MS is particularly sensitive to volatile solvents.

Green Chewing Gum
It’s not just suspected arson cases which make use of these techniques. In an unusual case involving a purpose-built crop-spraying machine worth hundreds of thousands of pounds, the interaction between a new formulation of additive and an otherwise normal spray mixture resulted in a dark green, chewing gum-like precipitate. This rapidly hardened to a plastic-like substance, resulting in the spray reservoir, spray lines, filters and spray nozzles becoming clogged. Analysis of the precipitate by X-ray spectroscopy revealed an extremely high level of copper – and there was only one source of copper to be found; in the newly formulated additive. Further analysis of the precipitate by FTIR identified a complex molecule similar in nature to a urea-formaldehyde polymer, the components of which had come from the regular spray mixture. Dark green granules were discovered in the additive, suggesting it had not been prepared correctly by the manufacturer, and it was concluded that these granules had acted as ‘seeds’ for precipitate formation. In this case, although the subsequent repair to the crop sprayer cost tens of thousands of pounds, it was not possible to confirm whether the problem arose from a manufacturing issue or incorrect storage by an intermediate supplier, and recovery action was not pursued.

Orange Bedrooms
What initially appeared to be a small fire with a simple explanation, consuming only the mattress of a bed, resulted in extensive testing for toxic contaminants. Everything in the bedroom was stained orange – the fittings, the furniture, and the carpet, to the extent that the room looked to have travelled through time from the 1970s. X-ray analysis was employed to analyse the orange deposits, which revealed unusually high levels of antimony and bromine, both of which can form orange salts. These elements are used in flame retardants, and this inert state, they do not pose a health risk, but they can generate highly toxic fumes and deposits if released in a fire. These deposits posed real health hazards, arising from either skin contact and subsequent transfer to the digestive system, or inhalation of dust. FTIR analysis also detected compounds consistent with glues used in the manufacture of the mattress. Hawkins was able to advise on safe cleanup procedures and revisited after remediation to confirm the bedroom was clean and free from contamination.

Summary
There are too many examples to list where experts at Hawkins have drawn on many different analytical techniques. Our expertise in sampling and the interpretation of the results is used to investigate the contamination of fuels, food and pharmaceuticals, through to environmental and soil sample testing. We are often able to offer more flexibility and expertise to our clients by working closely with accredited analytical laboratories, with whom we can discuss our specific requirements. This article is intended to share just some of the analytical techniques we employ at Hawkins to answer questions about complex matters and assist clients, policyholders and other parties. If you have the need for the services of a forensic scientist, get in touch for a free consultation.
About The Author
James completed his PhD researching a novel enzyme and its mechanism of action in blood cells, before undertaking six years of postdoctoral research into the biochemistry of various cellular signalling pathways. Whilst working at a small biotechnology company, James was responsible for the chemicals production business, serving hospitals throughout the UK and Europe with histological stains. He built up the product stewardship requirements of the business and qualified as a road transport Dangerous Goods Safety Adviser. He also worked as a Product Steward, assessing and assigning hazard and transport classifications to new and reformulated petrochemicals products, for Shell and Gulf Oil before joining Hawkins as a forensic investigator. James aids both the fire investigation and contamination teams at Hawkins, with his extensive knowledge of chemical reactions.
 Italiano
Italiano




